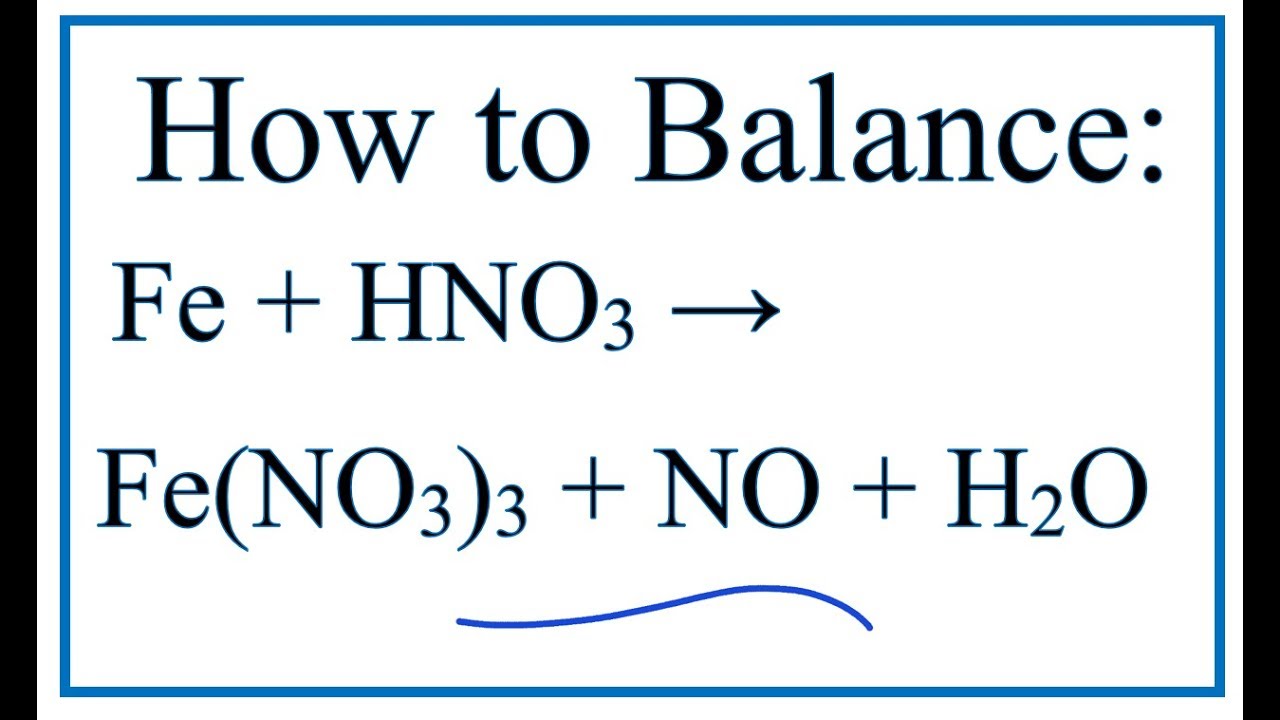
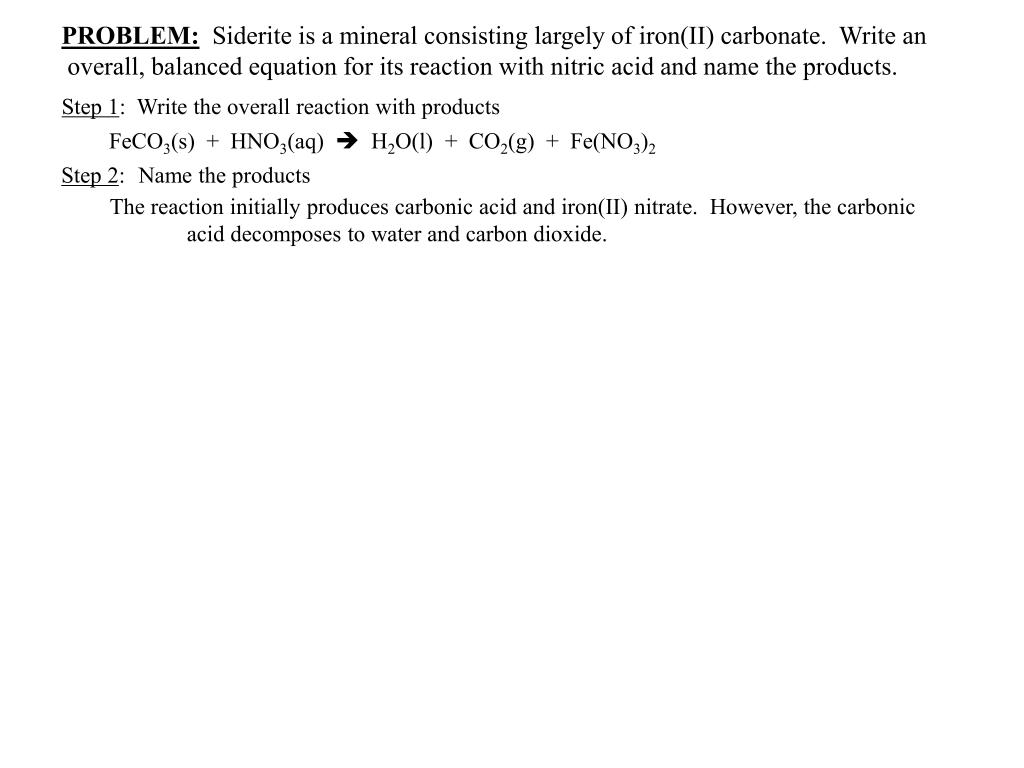
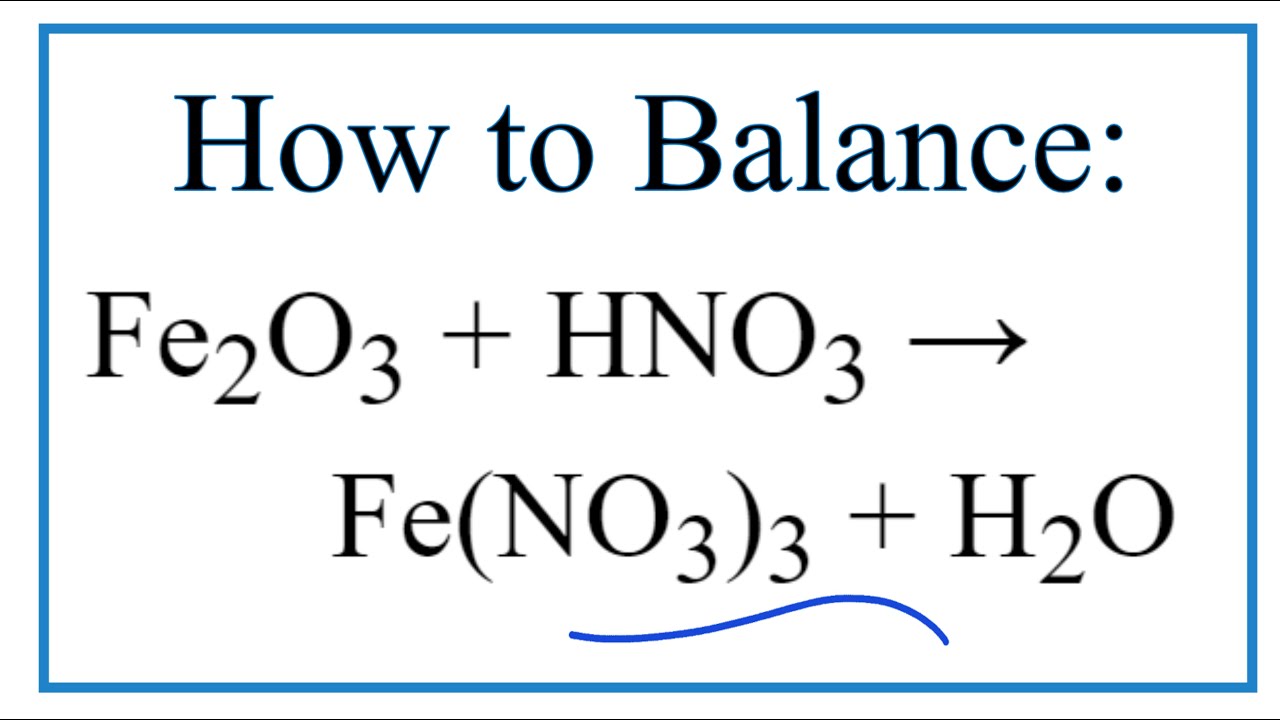
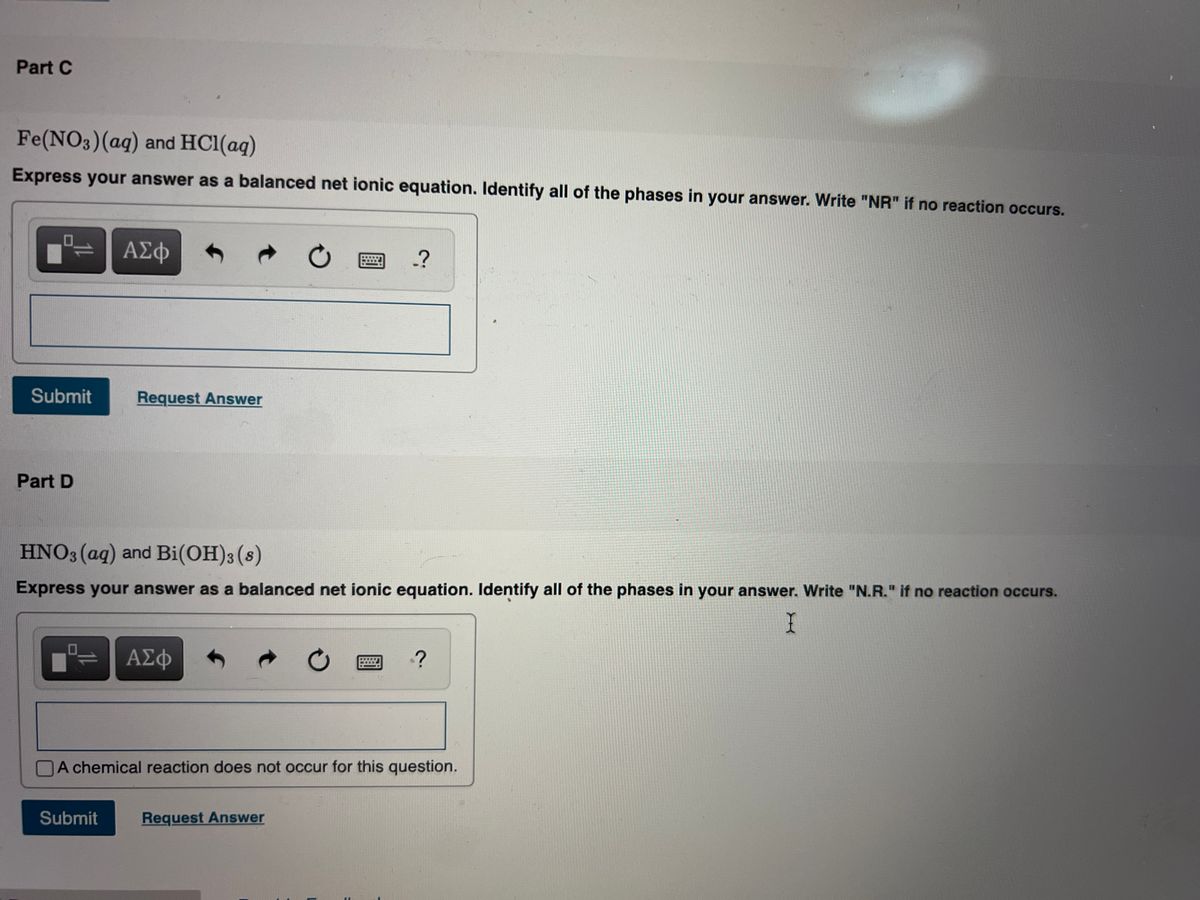
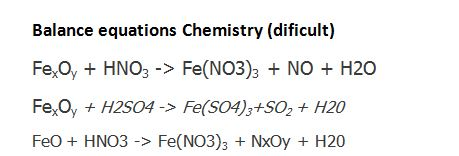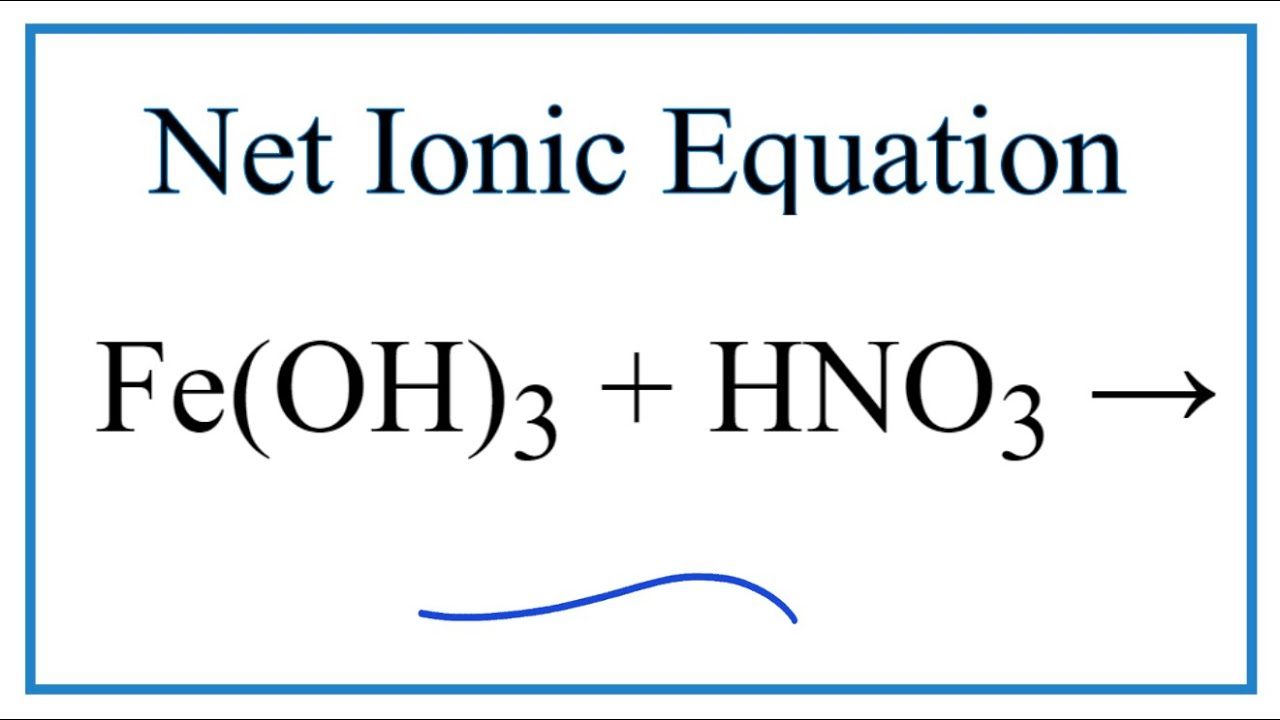SOLVED: Question 33 (2 points) When the following equation is balanced, what is the coefficient folllkz Fe HNO3 Fe(NO3)3 Hz 0
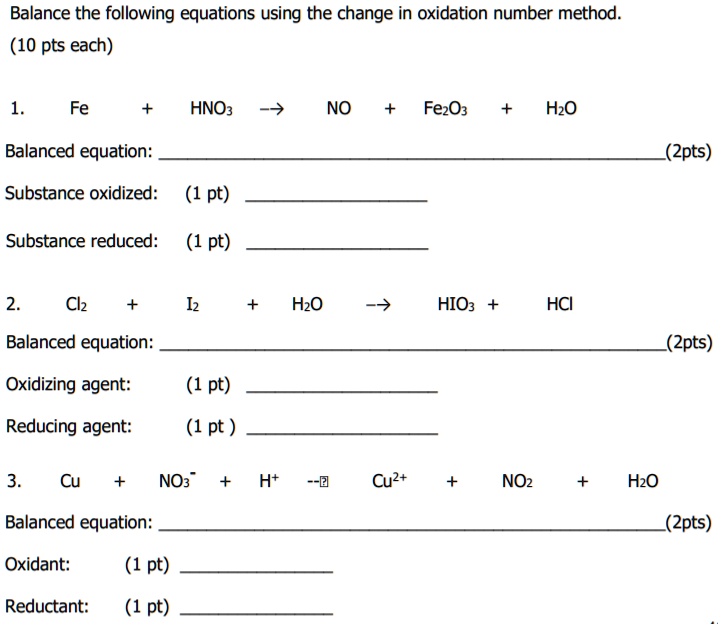
SOLVED: Balance the following equations using the change in oxidation number method (10 pts each) Fe HNOz NO FezOz Hzo Balanced equation: (Zpts) Substance oxidized: (1 pt) Substance reduced: (1 pt) Clz
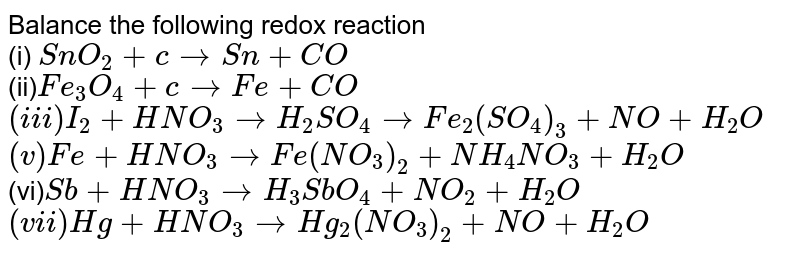
Balance the following redox reaction (i) SnO(2)+c toSn+CO (ii)Fe(3)O(4)+c to Fe +CO (iii) I(2)+HNO(3) to H(2)SO(4) to Fe(2)(SO(4))(3)+NO+H(2)O (v)Fe+HNO(3) to Fe(NO(3))(2)+NH(4)NO(3)+H(2)O (vi)Sb+HNO(3) to H(3)SbO(4)+NO(2)+H(2)O (vii) Hg+HNO(3) to Hg(2 ...

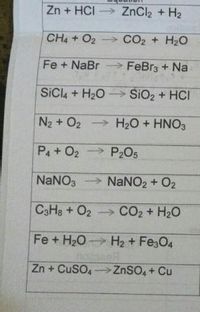
Balance the following equations a Fe H2O Fe3O4 H2 b Ca N2 Ca3N2 c Zn KOH K2ZnO2 H2 d Fe2O3 CO Fe CO2...
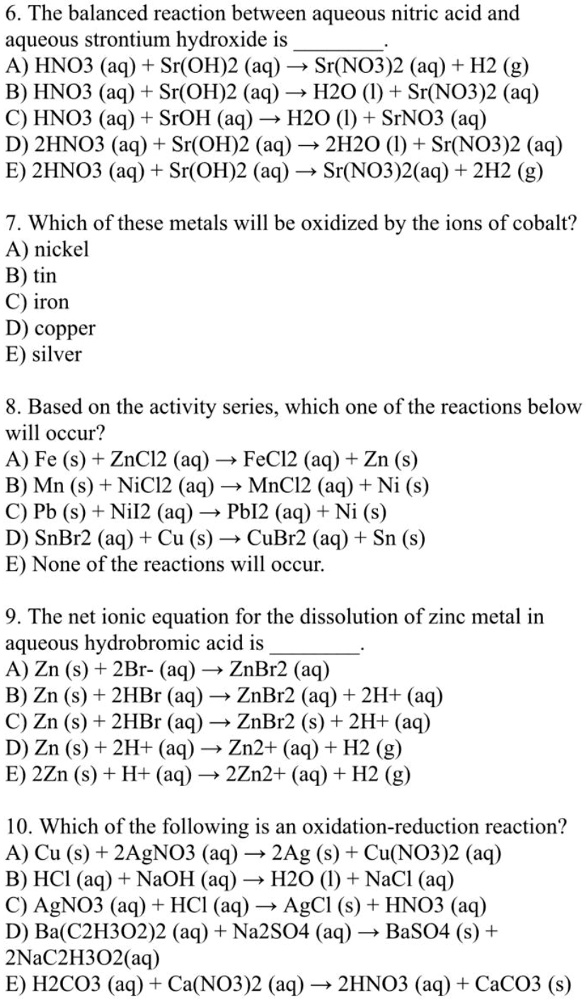
SOLVED: The balanced reaction between aqueous nitric acid and aqueous strontium hydroxide is HNO3 (aq) + Sr(OH)2 (aq) 57 Sr(NO3)2 (aq) + H2 (g) B) HNO3 (aq) + Sr(OH)2 (aq) 5 H2O (

The ratio of coefficient of HNO3,Fe (NO3)2 and NH4NO3 in the following redox equation, Fe + HNO3→Fe (NO3)2 + NH4NO3 + H2O in the balanced form will be?
![A. When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? [ ? ] F e 2 + + [ ? ] C I O 2 ? [ ? ] F e + [ ? ] C I O 3 ? B. Water appe | Homework.Study.com A. When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? [ ? ] F e 2 + + [ ? ] C I O 2 ? [ ? ] F e + [ ? ] C I O 3 ? B. Water appe | Homework.Study.com](https://homework.study.com/cimages/multimages/16/2_redox_balancing_11041037245791770796.png)
A. When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? [ ? ] F e 2 + + [ ? ] C I O 2 ? [ ? ] F e + [ ? ] C I O 3 ? B. Water appe | Homework.Study.com

Lakhmir Singh Chemistry Class 10 Solutions For Chapter 1 Chemical Reactions And Equations - Free PDF

Solve the following equation by using ion electron method Fe(NO3)2 + HNO3 = Fe(NO3)3 +NO + H2O - Brainly.in

Balance the given equation by oxidation number method - FeSO4 + HNO3 + H2SO4 = Fe(SO4)3 + NO + - Chemistry - Redox Reactions - 13629296 | Meritnation.com